The regulatory requirements for medical devices are increasing. Regulatory Affairs Managers ensure their company stays compliant with national, European, and international standards, laws, and regulations, securing market approval, compliance, and the competitiveness of healthcare products.
Regulatory Affairs – Medical Devices
Master of Science | Fürth
You can look forward to Regulatory Affairs: Pharmaceuticals and Drugs and Food!
You can look forward to Regulatory Affairs: Pharmaceuticals and Drugs and Food!

All Facts at a Glance
Study contents Regulatory Affairs, M.Sc.
From Bureaucratic Burden to Strategic Success
Regulatory Affairs is far more than just bureaucracy – it is the key to a successful market launch! Modern Regulatory Affairs Managers are evolving into strategic advisors for management. Only those who understand regulations and their impact on product approval can effectively access the right markets and position products efficiently.
You will gain comprehensive knowledge of medical device regulations, including the approval processes for medical devices and health technology products both nationally and internationally, particularly focusing on the European Medical Device Regulation (MDR) and FDA Approval. You will learn how approval processes for medical devices work on national and international levels and explore the strategies companies use to bring products to market efficiently and in compliance with legal requirements. A key focus is on aligning regulatory frameworks with business objectives, enabling innovation while maximizing market opportunities.
With the increasing role of digitalization and artificial intelligence, regulatory processes are evolving. During your studies, you will explore modern technologies that automate regulatory workflows and analyze how data-driven decision-making can accelerate approvals. You will also gain insights into risk management, quality management systems, and post-market surveillance, ensuring the safety and effectiveness of products throughout their entire lifecycle.
Through practical case studies and an in-depth examination of international regulations such as MDR, FDA, and ISO standards, you will be well-prepared to navigate regulatory challenges and develop effective solutions. This program not only equips you with technical expertise but also enhances your analytical and strategic skills, enabling you to successfully manage complex regulatory requirements in global healthcare markets.
Our range of study programmes in Health and Law is very diverse. Let yourself be inspired.
Career opportunities, Regulatory Affairs, M.Sc.
Your Future in the Regulation of Healthcare
A Master's in Regulatory Affairs opens up a wide range of career opportunities in medical technology, the pharmaceutical industry, and regulatory agencies. With your expertise, you will help bring innovative healthcare products to market efficiently and in full compliance with regulations.
Typical career paths are for example:
- Regulatory Affairs Manager for medical devices & pharmaceuticals
- Auditor or product expert for medical technology
- Quality or Compliance Manager in the healthcare industry
- Consultant for regulatory affairs & market strategy
- Project Manager for product approval & market entry
- Regulatory agency or certification body specialist
- Expert in post-market surveillance & risk management

- Free and without obligation
- Detailed information about your studies
- Event invitations and individual consultation

Your modules
In the Regulatory Affairs (M.Sc.) programme at SRH University, you will develop essential skills to thrive in the international medical technology industry. Beyond mastering regulatory requirements, you will learn how to seamlessly integrate them into the development of medical devices. Your studies will cover key areas such as quality management systems, international approval processes, and risk management – all crucial for ensuring safe and marketable products.
The modules cover key topics such as the implementation of quality management systems, global regulatory frameworks, and the requirements for clinical trials. You will gain hands-on expertise in post-market surveillance, health economics in market access, and the application of artificial intelligence (AI) in regulatory affairs. Additionally, you will explore current medical technology trends, ethical considerations, and strategic regulatory processes. Elective modules allow you to specialize in advanced verification and validation methods or strategic regulatory management, ensuring you are well-prepared for the evolving challenges in the medical technology sector.
We will be happy to send you further information material. You will also find the full curriculum there.
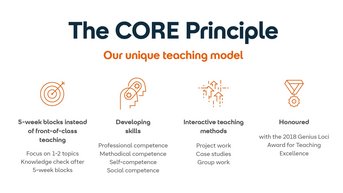
From study straight into practice
In addition to technical and methodological skills, social and personal skills are crucial today. SRH University 's innovative, successful and award-winning CORE principle promotes independent and active learning so that you can apply your knowledge directly in practice - for a clear competitive advantage.





